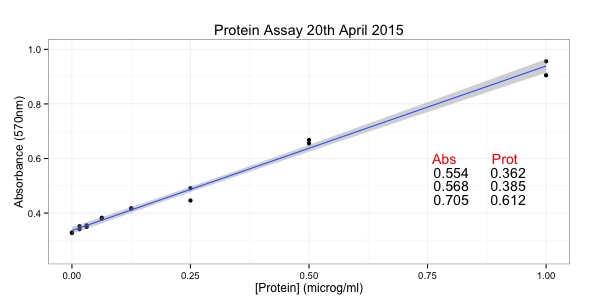
In my previous two scripts, I have looked at analysing an image step by step. However, what we really want to do is to analyse multiple images and run the same analysis across all of them. One good way to do this in R is to bring all the images into one object - a list.
This allows us to write functions and then use the lapply( ) function to apply it to each element of the list.
The script below downloads 48 images from a folder on Github. These images are turned grey to allow masking. Then the regions of fluorescence are identified and the number of regions found with each staining is determined. We can look at the distribution of cells through the different layers.
The layers detail the staining of cells in a Drosophila embryo with different methods. The include fluorescence markers and nuclear stains.
Then I have extracted some data into a data frame and drawn graphs with ggplot2. Here are the graphs:
The graphs imply that the cell number as determined by the nuclear staining (blue graph) are similar in all the layers. They layers are generated as the fly embryo is imaged horizontally. The numbers of cells detected with Stain number 1 also stays constant while the number of cells detected with Stain number 2 diminish as we transit down the embryo.
Here is an example of the images:
 |
| Three of the images from the stack of 48. Left hand panel (green) is stain 1, middle panel is nuclei staining, right hand panel (white) is stain 2. |
Here is the script. It's a bit long - 207 lines ! It might be easier to cut and paste from Github.
SCRIPT START
# install.packages("EBImage", "ggplot2", "RCurl") # if required.
library(EBImage)
library(ggplot2)
library(RCurl)
# this is a link to the list of files in our folider
link <- "https://raw.githubusercontent.com/brennanpincardiff/RforBiochemists/master/data/images/imageSeq/filenameslist"
# the download.file() function downloads and saves the file with the name given
download.file(url=link, destfile="filenameslist", mode="wb")
load("filenameslist")
# how many files are in this list?
length(filelist)
# 48
# this creates the first function called downloadImages
downloadImages <- function(x){
# assemble URLs
url <- paste0("https://raw.githubusercontent.com/brennanpincardiff/RforBiochemists/master/data/images/imageSeq/", x)
cat("Image downloaded") # so that I know something is happening.
img <- readImage(url)
}
# read in all the files into a list using the lapply( ) function
# lapply - applies a function to a list or a vector.
# in this case, it applies the function readImage() to each element in the
# object filelist
# lapply( ) syntax: name of list, name of function
images <- lapply(filelist, downloadImages)
# it takes a while to download 48 images - be patient
# we now have all 48 images in a list
# to show us that R knows this is a list we can use the function mode( )
mode(images) # output to console says "list"
# to access a single image in the list we use double square brackets
display(images[[2]]) # display the second image in the list
# we can display the image brighter
display(images[[2]]*4, method = "raster")
# it might be good to save this locally so that it doesn't need
# to be downloaded again
# CHECK where you want to save it first
# setwd("SomeWhereYouWantToSaveTheData")
# setwd("/Users/paulbrennan/Desktop") # example
save(images, file = "Dros_Images")
# this can then be reloaded using the load( ) function
load("Dros_Images")
# Next step to alter the list of images a a group
# make all the images greyscale
# http://stackoverflow.com/questions/20347025/convert-rgb-image-to-one-channel-gray-image-in-r-ebimage
# create another function...
makeGrey <- function(img){
print("One image grey")
channel(img, mode = "grey")
}
# then apply the function to the list of images using lapply
# lapply( ) syntax: (name of list, name of function)
images.grey <-lapply(images, makeGrey)
# we have now created a list of grey scale images.
display(images.grey[[2]]*4, method = "raster) # allows to check.
# write a function to create image masks
maskCells <- function(img1){
# blur the image
img1 <- gblur(img1*2, sigma = 5)
# apply a Otsu threshold
img1.mask <- img1 > otsu(img1)
# generate an image with different values for each connected region
# key here is the bwlabel( ) function
img1.mask <- bwlabel(img1.mask)
# show this as a coloured blobs
display(colorLabels(img1.mask), method = "raster")
# the bwlabel() function 'counts' the blobs
count <- max(bwlabel(img1.mask))
# this outputs the count to us
cat('Number of cells in this image =', count,'\n')
return(img1.mask)
}
# make the image masks using the function and the lapply
images.mask <- lapply(images.grey, maskCells)
# result is a list of image masks
# write function to extract number of objects masked
countRegions <- function(img1.mask){return(max(bwlabel(img1.mask)))}
# to extract numbers from the image masks use lapply and function again
numbers <- lapply(images.mask, countRegions)
# result is a list of 48 numbers in this case
# use the numbers to make some plots
# in order to graph the numbers we put them into a data frame
numb <- unlist(numbers)
df <- data.frame(numb)
df$type <- c("memb", "st1", "nuc", "st2")
df$layer <- c(1,1,1,1,2,2,2,2,3,3,3,3,4,4,4,4,5,5,5,5,6,6,6,6,7,7,7,7,8,8,8,8,9,9,9,9,
10,10,10,10,11,11,11,11,12,12,12,12)
df.st1 <- subset(df, df$type == "st1")
df.st2 <- subset(df, df$type == "st2")
df.nuc <- subset(df, df$type == "nuc")
df.2 <- as.data.frame(cbind(df.nuc$layer, df.nuc$numb, df.st1$numb, df.st2$numb))
colnames(df.2) <- c("layer", "nuc", "st1", "st2")
# using the dataframe, I am making plots of the number of stained cells across the various layers
# make the plot for the numbers of nuclei
nucHist <- ggplot(df.2,
aes(x=as.factor(layer),
y=nuc)) +
geom_bar(stat="identity", fill = "blue") +
ggtitle("Nuclei per layer") +
ylab("Number of regions") +
xlab("Layers from top to bottom") +
theme_bw()
# show the plot
nucHist
# make the plot for the numbers of regions stained with stain number 1
stain1Hist <- ggplot(df.2,
aes(x=as.factor(layer),
y=st1)) +
geom_bar(stat="identity", fill = "green") +
ggtitle("Stain number 1") +
ylab("Number of regions") +
xlab("Layers from top to bottom") +
theme_bw()
# make the plot for the numbers of regions stained with stain number 2
stain2Hist <- ggplot(df.2,
aes(x=as.factor(layer),
y=st2)) +
geom_bar(stat="identity", fill = "grey") +
ggtitle("Stain number 2") +
ylab("Number of regions") +
xlab("Layers from top to bottom") +
theme_bw()
# from: http://www.cookbook-r.com/Graphs/Multiple_graphs_on_one_page_(ggplot2)/
# Multiple plot function
#
# ggplot objects can be passed in ..., or to plotlist (as a list of ggplot objects)
# - cols: Number of columns in layout
# - layout: A matrix specifying the layout. If present, 'cols' is ignored.
#
# If the layout is something like matrix(c(1,2,3,3), nrow=2, byrow=TRUE),
# then plot 1 will go in the upper left, 2 will go in the upper right, and
# 3 will go all the way across the bottom.
#
multiplot <- function(..., plotlist=NULL, file, cols=1, layout=NULL) {
library(grid)
# Make a list from the ... arguments and plotlist
plots <- c(list(...), plotlist)
numPlots = length(plots)
# If layout is NULL, then use 'cols' to determine layout
if (is.null(layout)) {
# Make the panel
# ncol: Number of columns of plots
# nrow: Number of rows needed, calculated from # of cols
layout <- matrix(seq(1, cols * ceiling(numPlots/cols)),
ncol = cols, nrow = ceiling(numPlots/cols))
}
if (numPlots==1) {
print(plots[[1]])
} else {
# Set up the page
grid.newpage()
pushViewport(viewport(layout = grid.layout(nrow(layout), ncol(layout))))
# Make each plot, in the correct location
for (i in 1:numPlots) {
# Get the i,j matrix positions of the regions that contain this subplot
matchidx <- as.data.frame(which(layout == i, arr.ind = TRUE))
print(plots[[i]], vp = viewport(layout.pos.row = matchidx$row,
layout.pos.col = matchidx$col))
}
}
}
# run code above to make multiplot function first
multiplot(stain1Hist, nucHist, stain2Hist, cols = 3)
SCRIPT END